Particle Reactions
Combustion of the coal is at the heart of the boiler. The mass release and the heat release due to coal combustion must be known in both space and time in order to calculate the heat transfer to the walls. Coal combustion consists of three processes:
- Moisture evaporation
- Pyrolysis (called devolatilization)
- Char oxidation
In addition, the soot formed from the coal volatiles in fuel rich zones must be considered for an adequate description of the temperature in the near-burner region.
Coal Pyrolysis
Tom Fletcher, Andrew Richards
Pyrolysis is thermal decomposition of the coal, resulting in volatile gases and solid char particles. Between 35 and 60% of the dry, ash-free coal is pyrolyzed and released as volatiles, depending on the type of coal. This process may take as little as 2-5 ms in a large boiler. A large portion of the volatiles are high molecular weight and hence condensable at room temperature. These high molecular weight volatiles are called tar. The tar can form soot via polymerization, or can be oxidized.
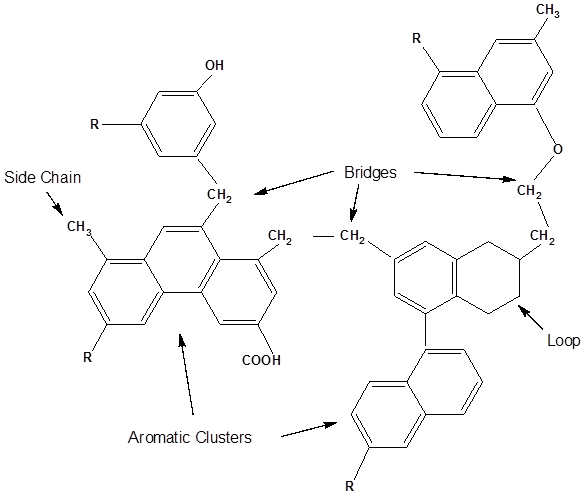 A cartoon of a portion of a hypothetical coal molecule
A cartoon of a portion of a hypothetical coal molecule
The chemical percolation devolatilization (CPD) model describes pyrolysis based on the chemical structure of the coal. Some of the features of the CPD model are shown below:
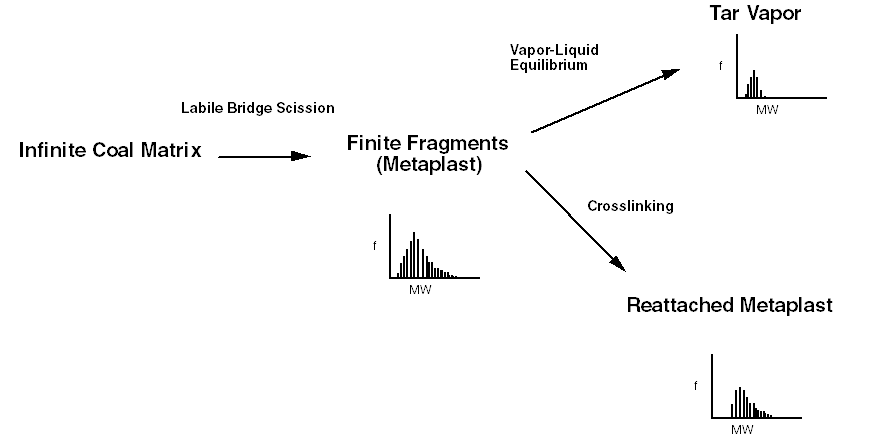 Processes described by the CPD model
Processes described by the CPD model
This research involves implementation of many of the features of the CPD model into the LES simulations, as well as performing the V-UQ analysis for the CPD model.
Char Oxidation
Tom Fletcher, Troy Holland
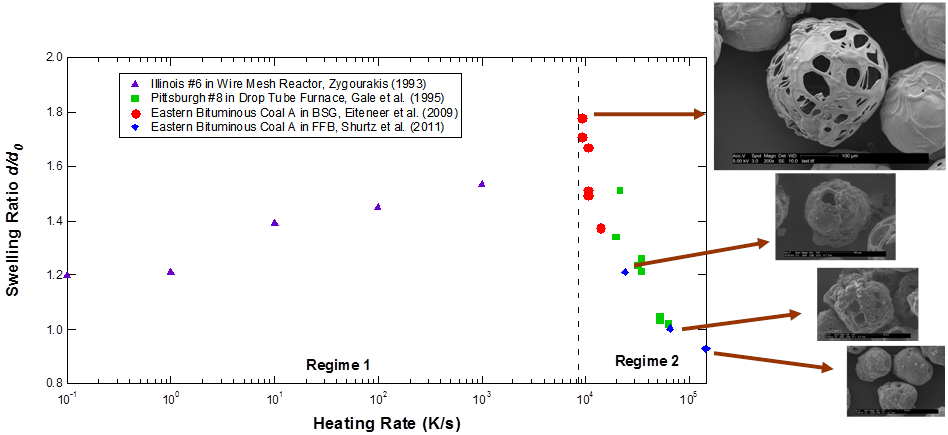
The solid particles remaining after pyrolysis are called char. Char reactions for pulverized coal particles typically take about 1 s in an industrial boiler. The size, reactivity, and pore structure of the chars are very dependent on coal type as well as heating conditions during pyrolysis. For example, the char diameter after pyrolysis is a function of the maximum particle heating rate during devolatilization, as shown below:
Char particle diameters after pyrolysis vs. maximum heating rate during pyrolysis
(Figure taken from Shurtz, 2011)
Char is normally oxidized by O2, but in an oxy-fuel environment the char may also react with CO2, according to the following reactions:
C(s) + ½ O2 → CO
C(s) + O2 → CO2
C(s) + CO2 → 2 CO
Note that the heat of reaction changes greatly depending on which reactions occur. Diffusion of oxidizers through the particle boundary layer must be modeled, as well as diffusion of products away from the particle surface:
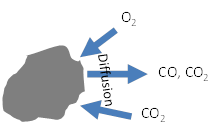
Diffusion of gases through char particle boundary layer
Diffusion of gases into pores in the char may also important and must be modeled. The LES model for the large simulation will not be able to resolve particle boundary layers. Char diameter and density change during reaction and must be described. Fragmentation may occur as the char burns out.
Soot from Coal
David Lignell, Tom Fletcher
Soot formation in coal systems comes largely from coal tar, unlike soot formed in flames from light hydrocarbons such as methane or propane. The tar molecular weight is highly aromatic and has an average molecular weight of about 350 amu. Laboratory experiments have shown that the soot yield at elevated temperatures matches the tar yield. Soot burns in the flame and transfers heat via radiation in the near-burner region of the boiler.
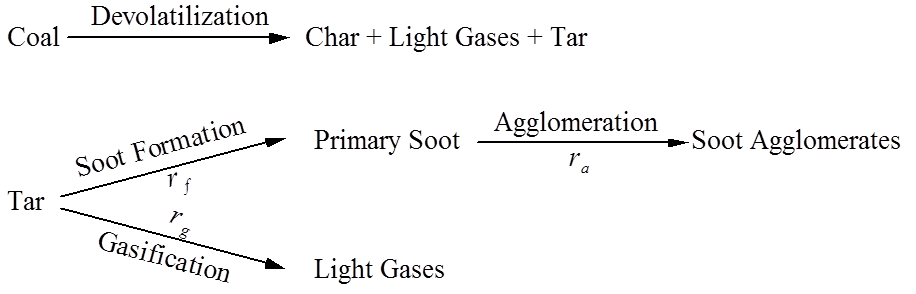
Soot formation, agglomeration, and oxidation must be modeled, and a postulated pathway is shown below:
(see Brown and Fletcher, 1998)
References
- Brown, A. L. and T. H. Fletcher, "Modeling Soot Derived from Pulverized Coal," Energy and Fuels, 12, 745-757 (1998).
- Eiteneer, B., R. Subramanian, S. Maghzi, C. Zeng, X. Guo, Y. Long, L. Chen, R. JS, A. Raman, J. Jain, T. Fletcher and R. Shurtz, "Gasification Kinetics: Modeling Tools Development and Validation," 26th Annual International Pittsburgh Coal Conference, Pittsburgh, PA (2009).
- Gale, T. K., C. H. Bartholomew and T. H. Fletcher, "Decreases in the Swelling and Porosity of Bituminous Coals during Devolatilization at High Heating Rates," Combustion and Flame, 100(1-2), 94-100 (1995).
- Shurtz, R. C., "Effects of Pressure on the Properties of Coal Char under Gasification Conditions at High Heating Rates," Ph.D. Dissertation, Chemical Engineering Department, Brigham Young University (December, 2011).
- Shurtz, R. C., K. K. Kolste, and T. H. Fletcher, "A Coal Swelling Model for CFD Applications at High Heating Rates," Energy and Fuels, 25, 2163-2173 (2011).
- Zygourakis, K., "Effect of Pyrolysis Conditions on the Macropore Structure of Coal-Derived Chars," Energy & Fuels, 7(1), 33-41 (1993).